Alzheimer's single-nucleus endothelial cell transcriptomics
Background on endothelial cells and cerebral vasculature
Endothelial cells are a type of vascular cell that form a critical component of the ‘blood-brain barrier’, a dynamic structure that also includes pericytes and astrocytic endfeet to protect the brain and its ‘privileged’ immune system from materials in the peripheral bloodstream. They are long, oblique cells that adhere to each other to form the inner lining of capillaries and larger vessels, as pictured below:.
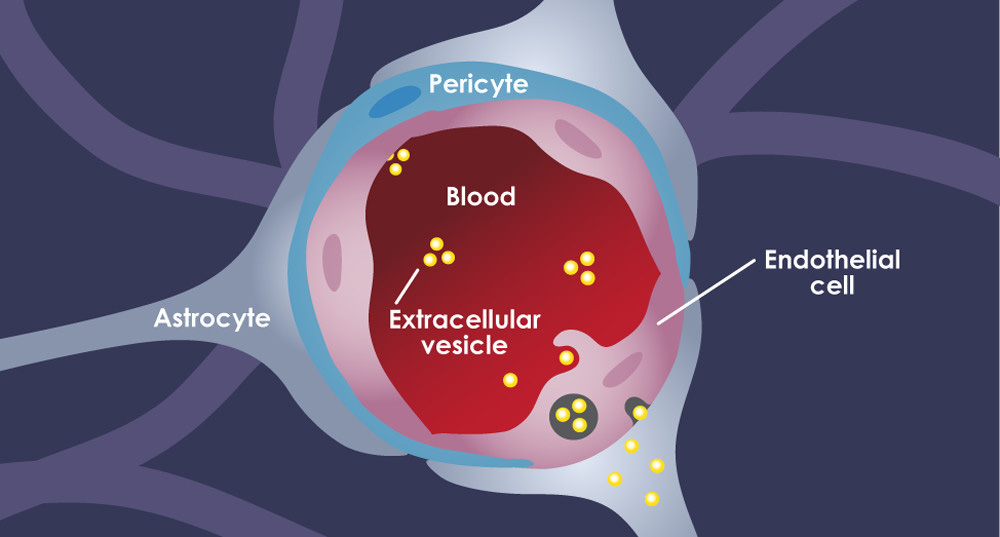
Vasculature and Alzheimer’s disease (AD)
Attention is increasingly being paid to the role of the cerebral vasculature in Alzheimer’s disease, a neurodegenerative disease that is pathologically characterized by the accumulation of intracellular tau neurofibrillary tangles and extracellular amyloid-β plaques. Mounting evidence suggests that changes to blood flow and the function of this blood-brain barrier can further promote these neurodegenerative pathways as well as cognitive decline. This seems to be a cycle, as AD pathology can in turn drive deleterious changes in the cerebral vasculature, including dysfunctional blood vessel formation (‘angiogenesis’), inflammation, excess removal of existing vessels, and senescence.

Since neuronal loss is more statistically associated with tau tangles than with amyloid plaques and tau pathology alone can lead to morphological and functional alterations to the cerebral vasculature, we set our sights on the intersection between tau tangles and vascular dysfunction in the AD brain. We previously showed that biomarkers of cellular senescence and adhesion are transcriptomically upregulated in microvessels (e.g., capillaries) isolated from the dorsolateral prefrontal cortex of human donor brains with high tau tangle burdens:
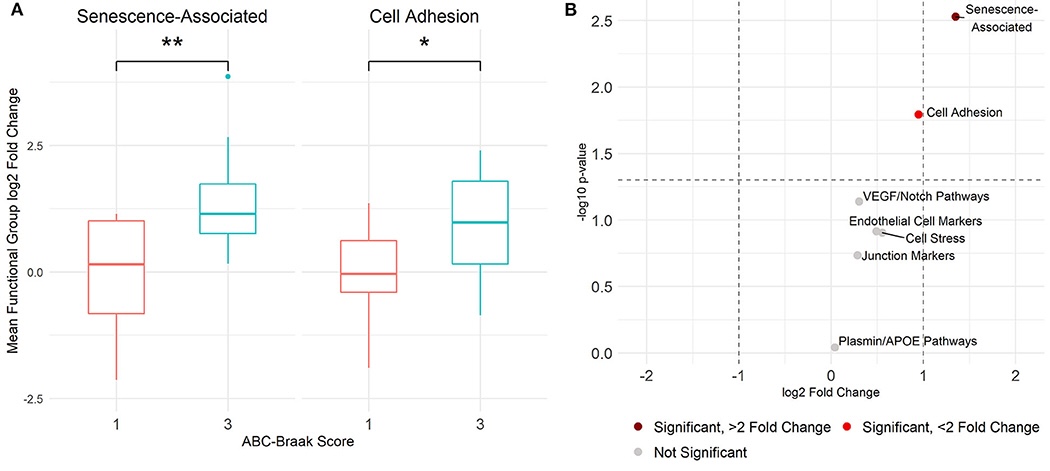
This was an important first step in demonstrating the tau-associated transcriptomic alterations to the human cortical vasculature. However, we know that endothelial cells differ in their molecular profiles and functions not only across organs, but also across regions within the cerebral cortex in both health and diseaes. It’s really important to understand how endothelial cells differ across the brain, because AD neuropathology affects brain regions differently; for example, medial temporal regions are most susceptible to early tau pathology, while areas like the primary visual cortex seem robust to pathology until very late stages of disease progression (Braak and Braak, 1991; Braak et al., 2006).
Methods in this approach
Considering that cortical brain areas are differently affected by amyloid β and tau pathologies, and that vascular changes are intimately related to AD, the goals of this work were twofold: (1) to characterize baseline heterogeneity in the endothelial cell transcriptome in the normal aged brain; and (2) to elucidate how regional differences in disease progression impact endothelial cell gene expression in AD. We performed single-nucleus RNA sequencing (RNAseq) on tissue from 32 human donors () with five brain regions in total, listed in order of highest to lowest tau pathology burden: entorhinal cortex, inferior temporal gyrus, prefrontal cortex, visual association cortex, and primary visual cortex.
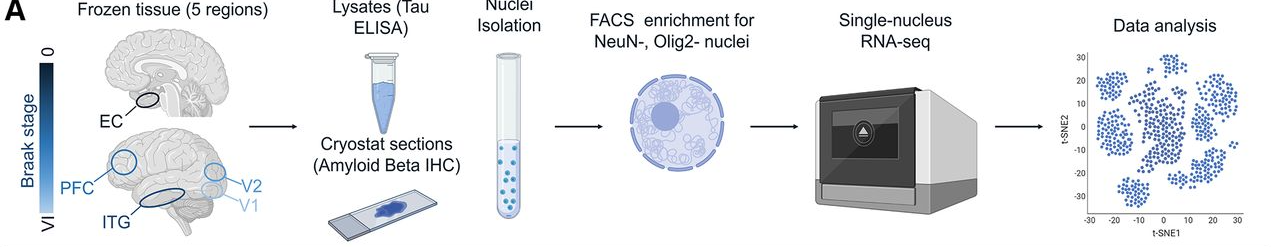
While we applied a coarse cell-sorting approach to mostly separate out neurons and oligodendrocytes for a separate analysis, the remaining nuclei comprised a heterogeneous mix of cell types, including endothelial cells, astrocytes, and microglia. To specifically identify endothelial cells, we used the Seurat R package to highlight canonical transcriptomic markers of endothelial cells:
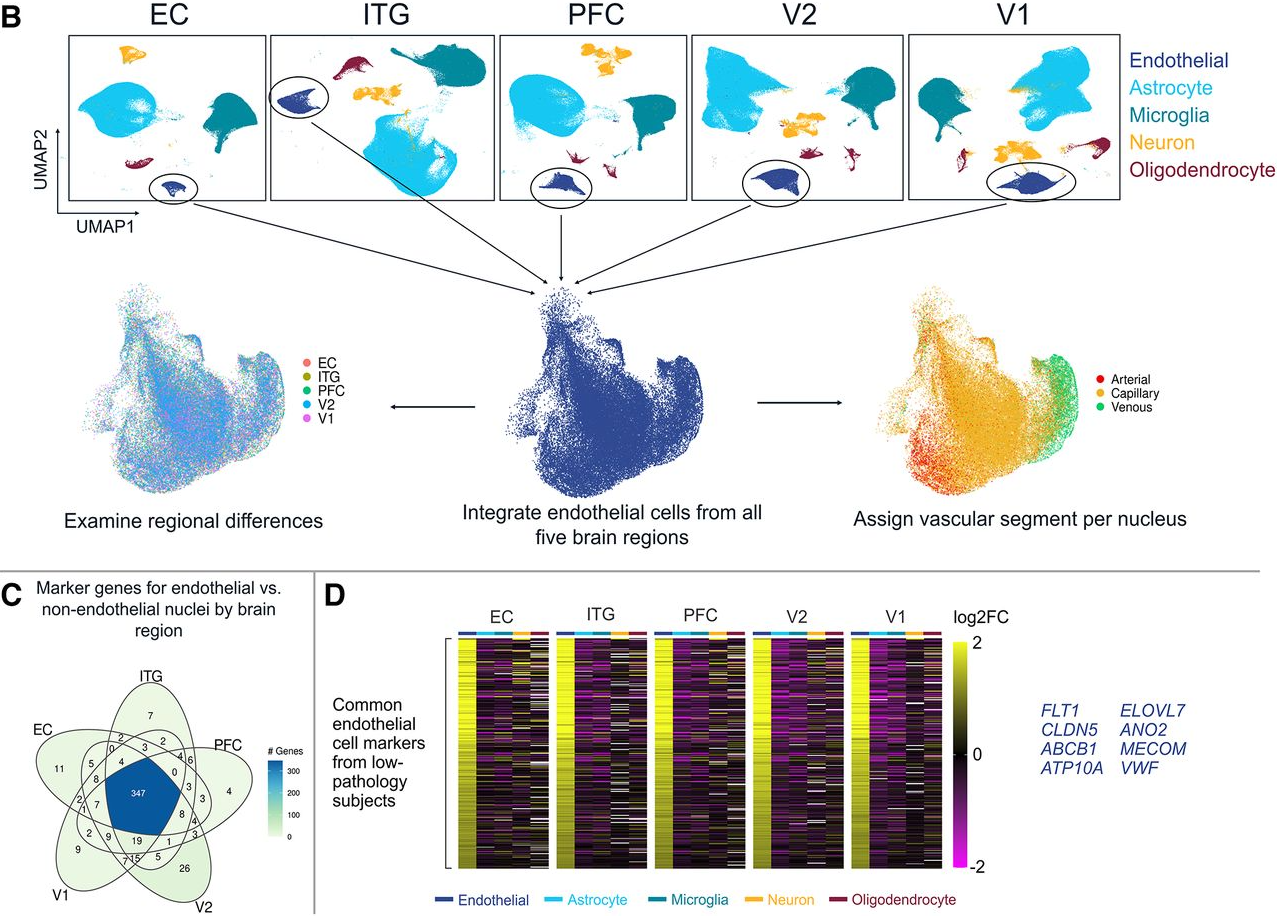
Inter-regional vascular heterogeneity in the healthy brain
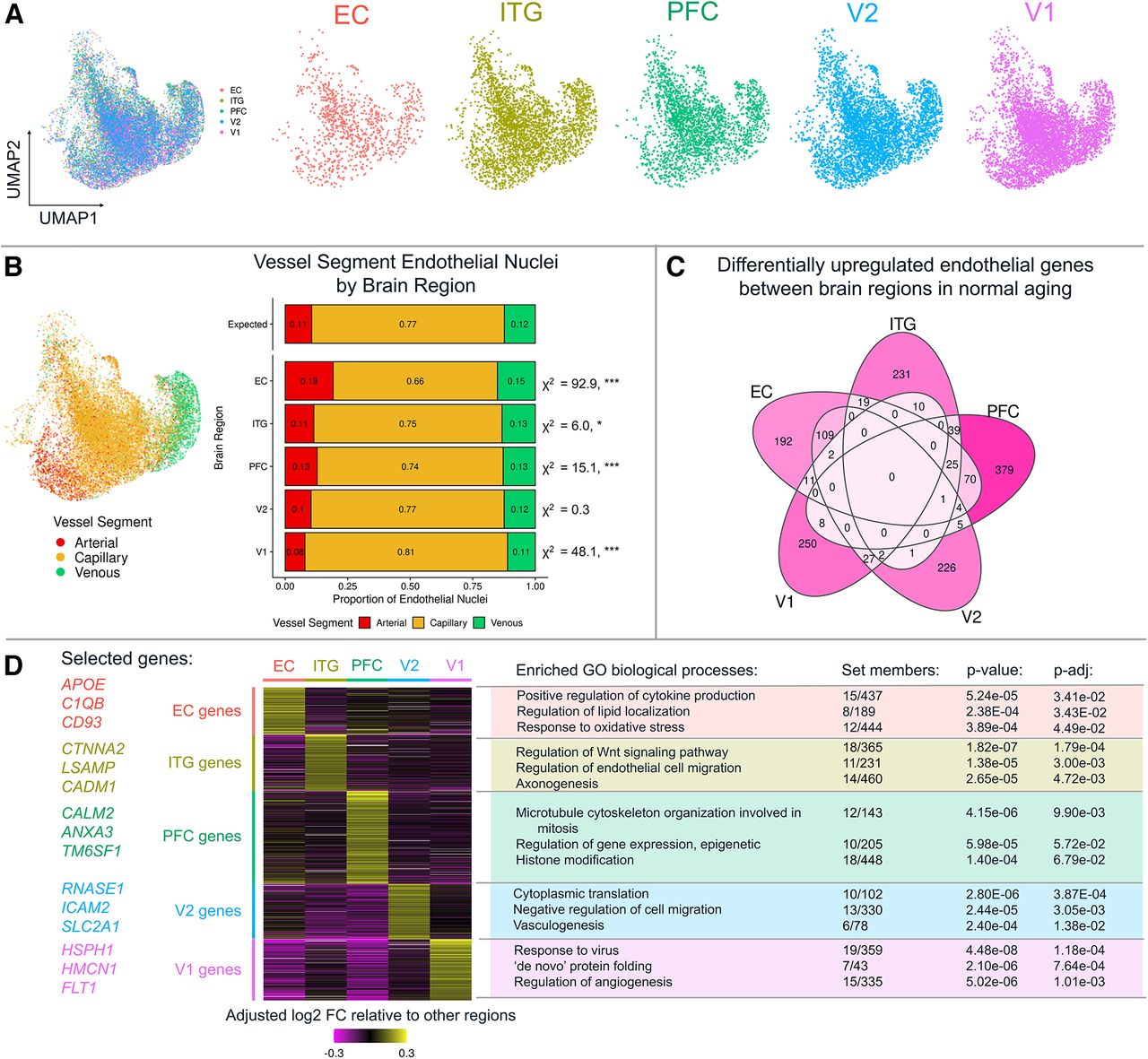
After identifying nuclei corresponding to endothelial cells, we focused on inter-regional differences across the five brain areas included in this experiment. Interestingly, the dimensionality reduction approach UMAP (uniform manifold approximation projection) largely separated nuclei based on vascular segment type (artery, capillary, or vein) rather than based on cortical region, as shown in Figure 5A-B below. Even so, we found hundreds of region-specific endothelial genes within the healthy donors, mapping to a set of distinctive biological pathways that include cytokine production (entorhinal cortex, EC), endothelial cell migration (inferior temporal gyrus, ITG), vasculogenesis (secondary visual cortex, V2) and ‘de novo’ protein folding (primary visual cortex, V1).
Whole-brain AD-related transcriptomic changes to endothelial cells
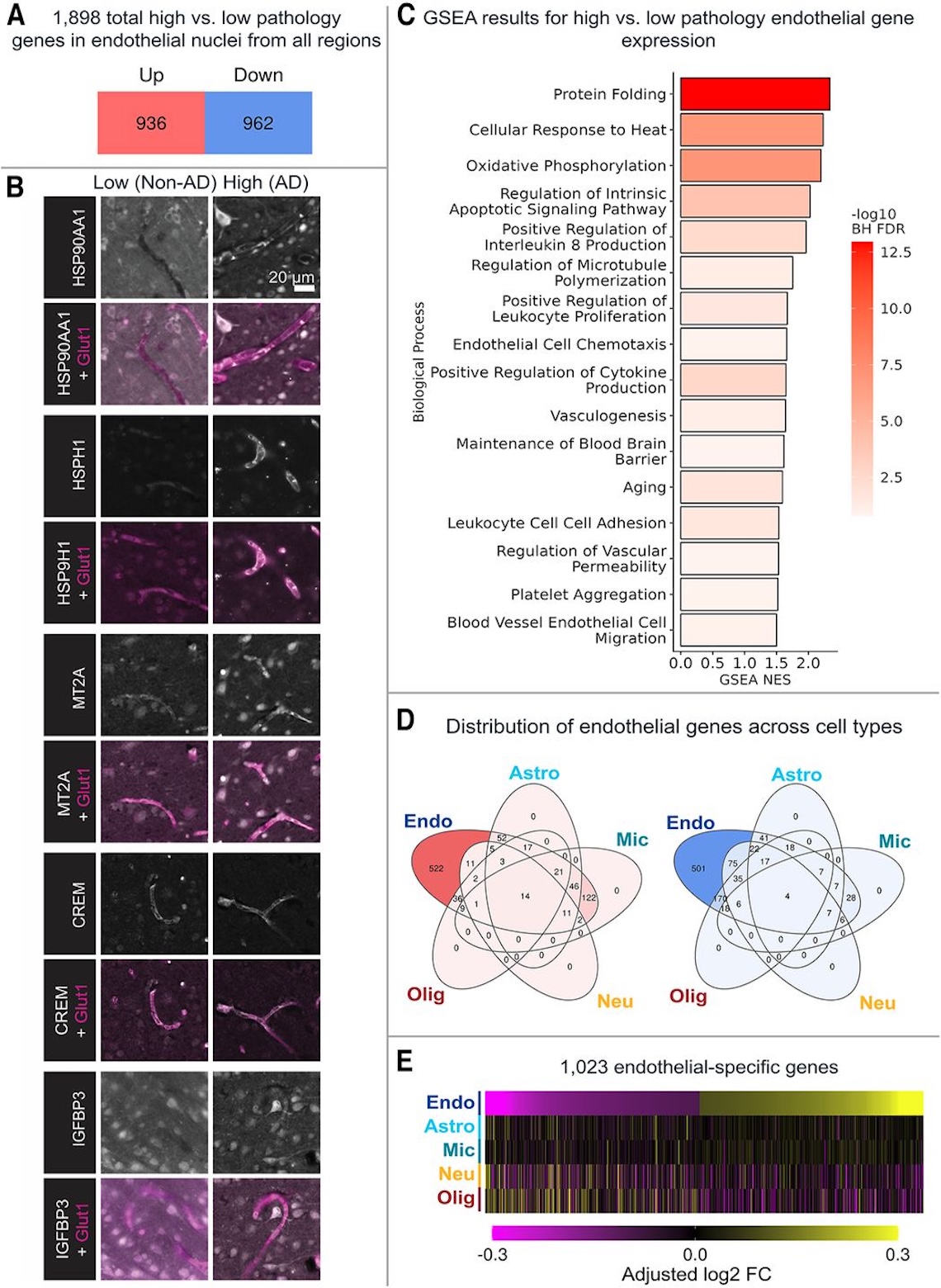
We next asked what transcriptomic markers are upregulated in endothelial cells across the AD brain, finding that 936 genes were upregulated and 962 downregulated brain-wide; of these, 522 upregulated genes are endothelial cell-specific, and 501 downregulated genes are endothelial-cell specific. Since these are just gene expression data, we confirmed localized protein expression of representative upregulated markers using immunofluorescence in Fig. 6B (shown below). We also performed gene-set enrichment analysis (GSEA) to identify common pathways that unify the upregulated genes, finding that high-tau endothelial cells are enriched for pathways including protein folding, cytokine production, leukocyte adhesion, and blood-brain barrier maintenance.
Region-specific vascular changes in AD
Zooming in to the regional level, we found that the most upregulated gene pathways (listed above) are shared across all five examined regions, as shown below in Figure 7. At the same time, something surprising is that the overall number of differentially expressed genes was much higher for V1 and V2 (in both directions), which was unexpected given that these regions are among the least susceptible to tau pathology in general.

Take-home messages
Using a large dataset of endothelial cell nuclei, the transcriptomic analysis that we report reveals new insights into regional gene expression differences in normal aged brain and AD. These data indicate that endothelial cells from different cortical areas are clearly distinguishable and are characterized by the expression of different marker genes and biological pathways. We also observed that AD pathology affects gene expression in brain endothelial cells, particularly those that make up capillaries. By comparing across donor samples from multiple disease stages, we observed the temporal relationship between pathology and endothelial transcriptome changes. Further, using these data, we compared the extent that AD-related factors, including plaques, CAA, tau, and APOE genotype, impact this cell type and examined how previously reported transcriptomic signatures vary between brain regions.